












Nous avons le plaisir d'annoncer que CODAN a établi un partenariat avec Sunnic Lighthouse GmbH, garantissant un approvisionnement en énergie 100 % verte provenant de parcs solaires.
Ce partenariat innovant associe un contrat d'achat d'électricité à un modèle d'approvisionnement complet. Il nous permet de bénéficier de prix d'électricité stables sur le long terme tout en éliminant les risques liés aux fluctuations de la demande énergétique ...en savoir plus (lien externe)

Nous avons le plaisir d'annoncer que CODAN a établi un partenariat avec Sunnic Lighthouse GmbH, garantissant un approvisionnement en énergie 100 % verte provenant de parcs solaires.
Ce partenariat innovant associe un contrat d'achat d'électricité à un modèle d'approvisionnement complet. Il nous permet de bénéficier de prix d'électricité stables sur le long terme tout en éliminant les risques liés aux fluctuations de la demande énergétique... en savoir plus (lien externe)
La gamme de seringues non stériles CODAN Oral se compose de seringues à usage unique fiables, précises et de haute qualité. Elle comprend une gamme pour administration orale à embout buccal avec un corps transparent et un corps ambré pour la protection des médicaments sensibles contre la lumière UV.
Les seringues CODAN Oral répondent également ...en savoir plus

La gamme de seringues non stériles CODAN Oral se compose de seringues à usage unique fiables, précises et de haute qualité. Elle comprend une gamme pour administration orale à embout buccal avec un corps transparent et un corps ambré pour la protection des médicaments sensibles contre la lumière UV.
Les seringues CODAN Oral répondent également ...en savoir plus
Le système de prélèvement sanguin XL a été conçu spécialement pour les applications pratiquées durant une mesure invasive de la pression sanguine.
Associés aux capteurs de pression précis et fiables de la série Xtrans®, les systèmes de prélèvement sanguin XL comportent des avantages exceptionnels...en savoir plus
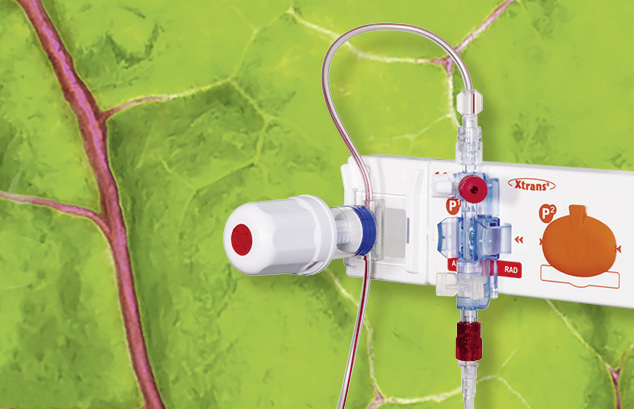
Le système de prélèvement sanguin XL a été conçu spécialement pour les applications pratiquées durant une mesure invasive de la pression sanguine.
Associés aux capteurs de pression précis et fiables de la série Xtrans®, les systèmes de prélèvement sanguin XL comportent des avantages exceptionnels...en savoir plus

